Q-Sens COVID-19 Detection Kit V2
Negotiable Min Order Quantity Unit
- Required Quantity
-
- Place of Origin
- South Korea
- Brand name
- Q-Sens® COVID-19 Detection Kit V2
- Payment Terms
- Negotiable
- Production method
- Negotiable
- Shipping / Lead Time
- Negotiable / Negotiable
- Keyword
- diagnosic kits, pcr test kit, covid-19

Cancer Rop Co., Ltd.
- Country / Year Established
-
 South Korea
/
2001
South Korea
/
2001
- Business type
- Manufacturer
- Verified Certificate
-
4




| Product name | Q-Sens COVID-19 Detection Kit V2 | Certification | CE |
|---|---|---|---|
| Category | Other Monitoring & Diagnostic Equipment | Ingredients | - |
| Keyword | diagnosic kits , pcr test kit , covid-19 | Unit Size | 94.0 * 62.0 * 55.0 mm |
| Brand name | Q-Sens® COVID-19 Detection Kit V2 | Unit Weigh | - |
| origin | South Korea | Stock | - |
| Supply type | - | HS code | - |
Product Information
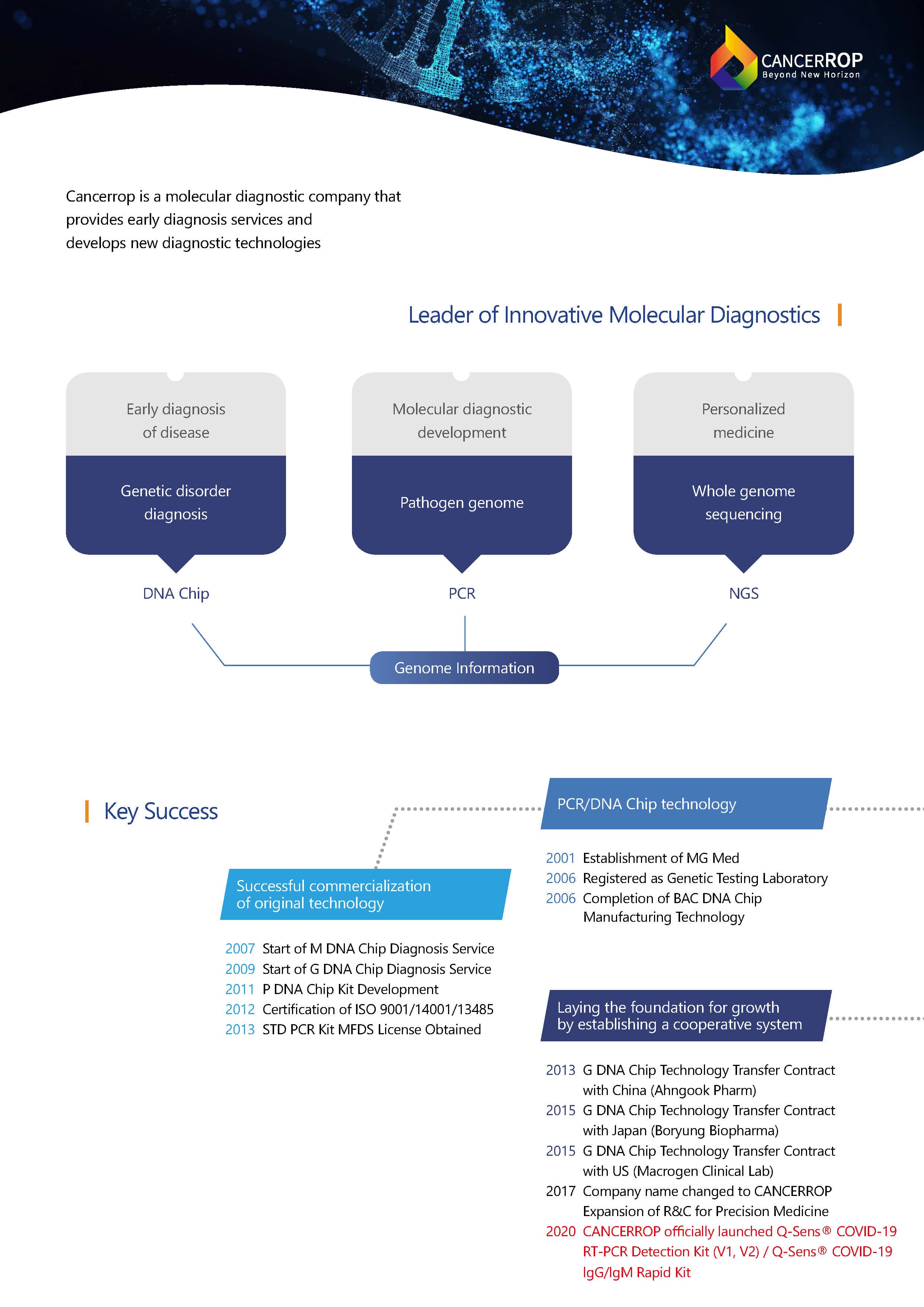
1.COMPANY PROFILE
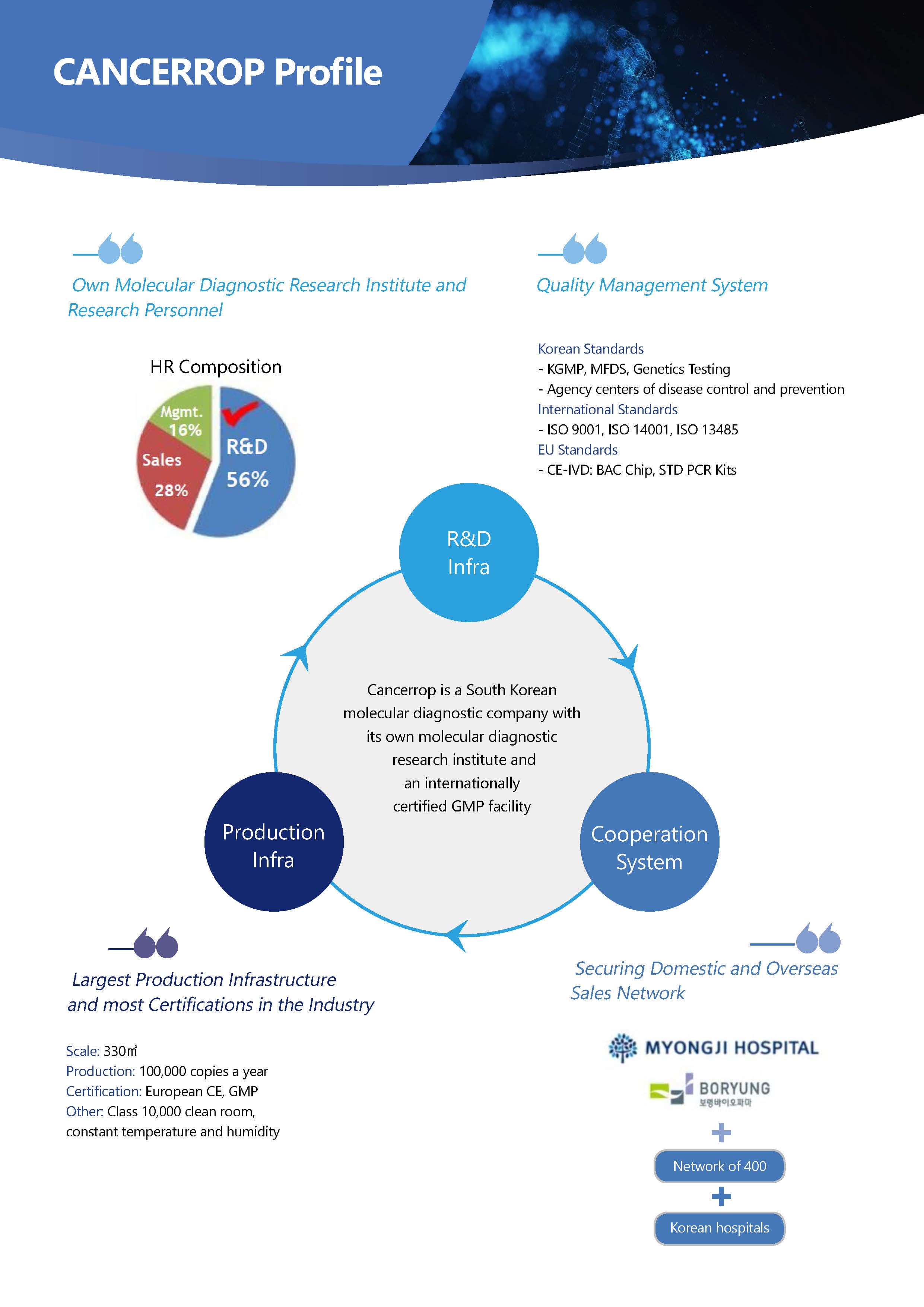
Cancerrop is a molecular diagnostic company with its own research institute and an internationally certified GMP facility, which is the largest production infrasttructure and most certifications in the industry.
..............................................................................................................................................................
...............
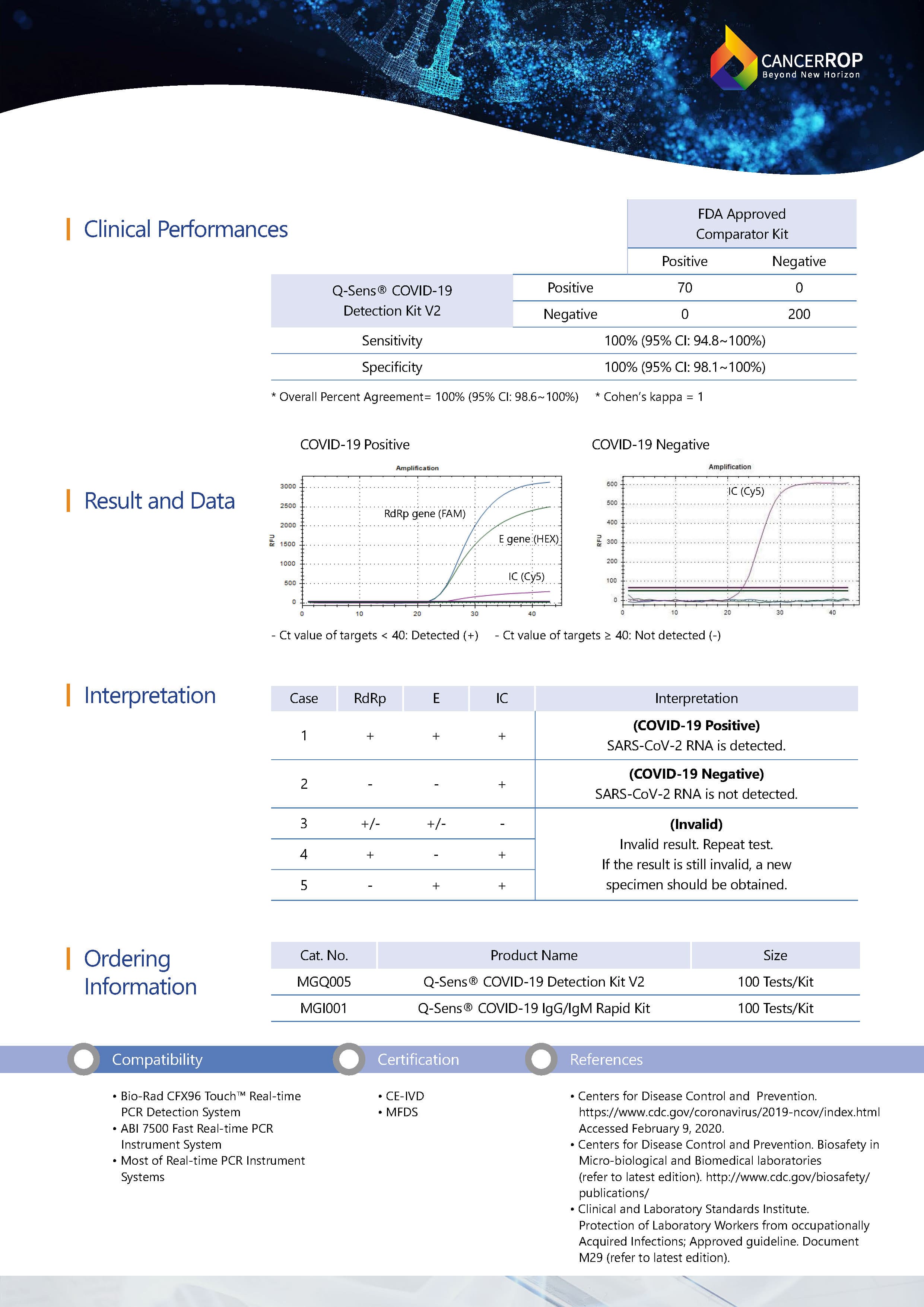
Q-Sens® COVID-19 RT-PCR Detection Kit V2
2. PRODUCT PROFILE
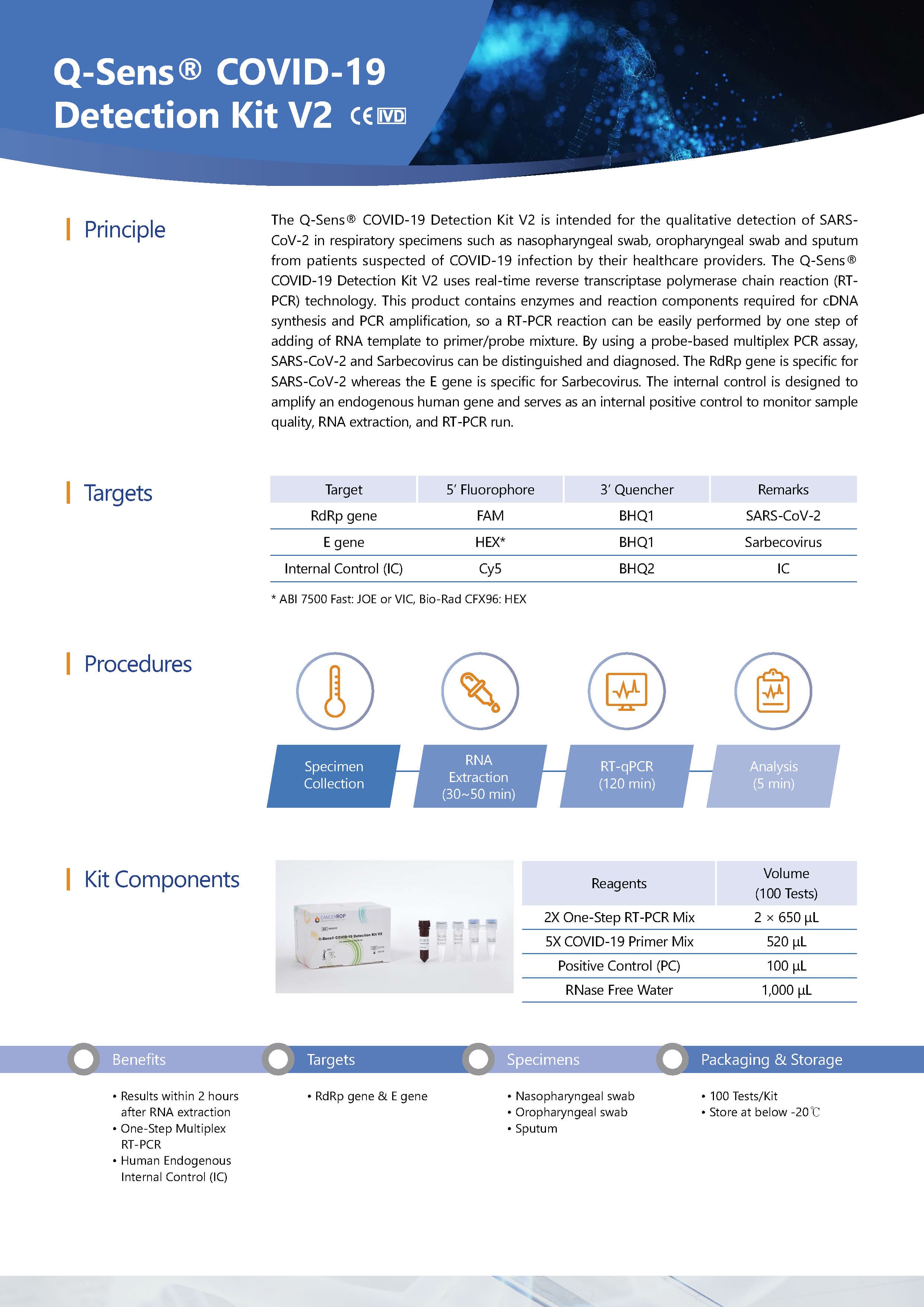
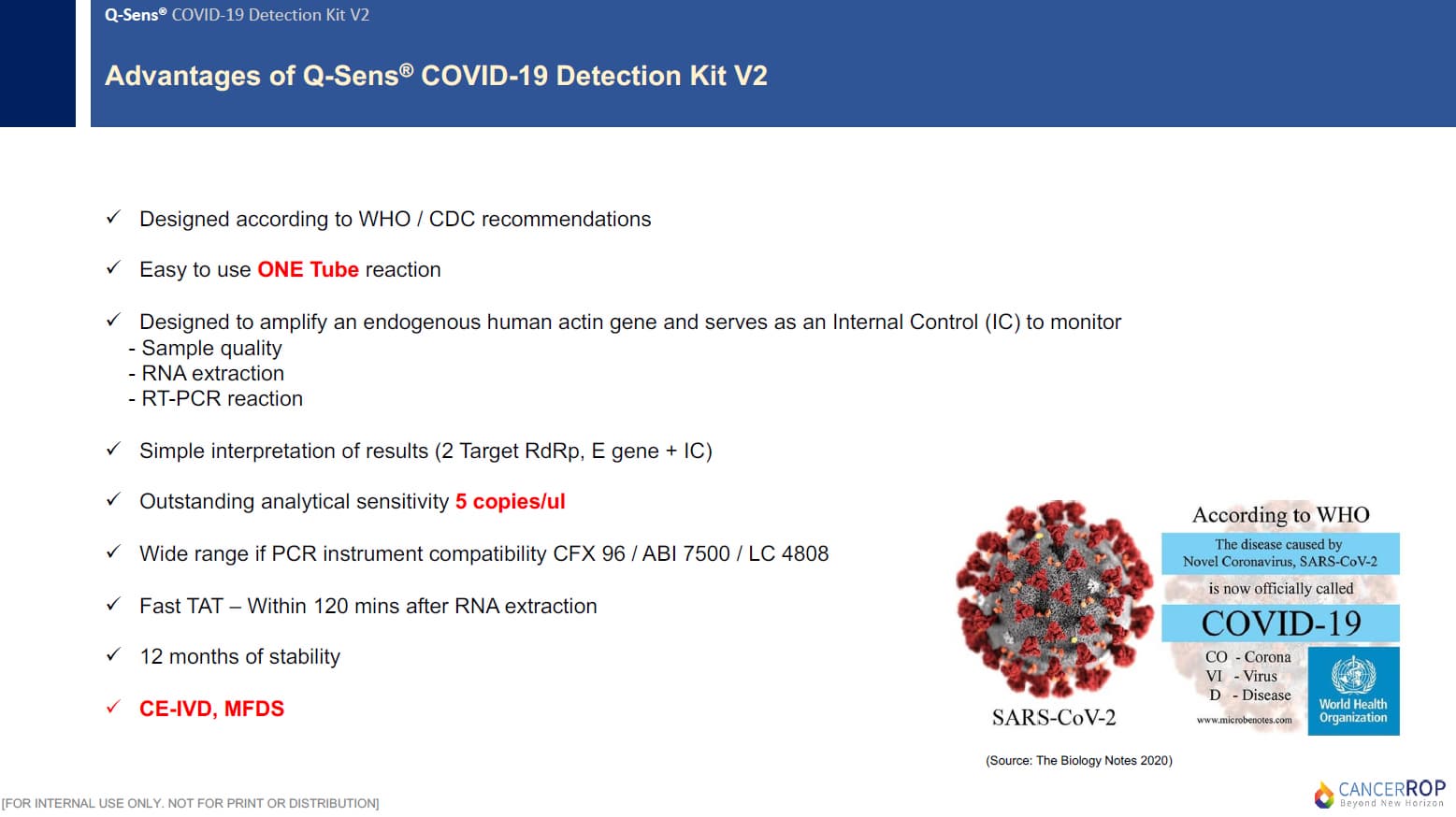
This Q-Sens® COVID-19 Detection Kit V2 uses real-time reverse transcriptase polymerase chain reaction(RT-PCR) technology. This product contains enzymes and reaction components required for cDNA syntesis and PCR amplification, so a RT-PCR reaction can be easily performed by one step of adding of RNA template to primer/probe mixture. By using a probe-based multiplex PCR assay, SARS-CoV-2 and Sarbecovirus can be distinguished and diagnosed. The RdRp gene is specific for SARS-CoV-2 whereas the E gene is specific for Sarbecovirus. The internal control is designed to amplify an endogenous human gene and serves as an internal positive control to monitor sample quality, RNA extraction, and RT-PCR run.
- Product Info Attached File
- Verified Certificate
-

B2B Trade
| Price (FOB) | Negotiable | transportation | - |
|---|---|---|---|
| MOQ | Negotiable | Leadtime | Negotiable |
| Payment Options | Negotiable | Shipping time | Negotiable |

Cancer Rop Co., Ltd.
- Country / Year Established
-
 South Korea
/
2001
South Korea
/
2001
- Business type
- Manufacturer
-
4




- President
- LEE WANG JUN
- Address
- Geumcheon-gu, Geumcheon-gu, Seoul, Korea
- Product Category
- Other Monitoring & Diagnostic Equipment
- Year Established
- 2001
- No. of Total Employees
- 51-100
- Company introduction
-
1.COMPANY PROFILE


2. PRODUCT PROFILE
Q-Sens® COVID-19 RT-PCR Detection Kit V2
2-1. PRODUCT DESCRIPTION


Q-Sens® COVID-19 RT-PCR Detection Kit V2
2-2. PRODUCT FEATURES
Q-Sens® COVID-19 IgG/IgM Rapid Test Kit
2-1. PRODUCT DESCRIPTION

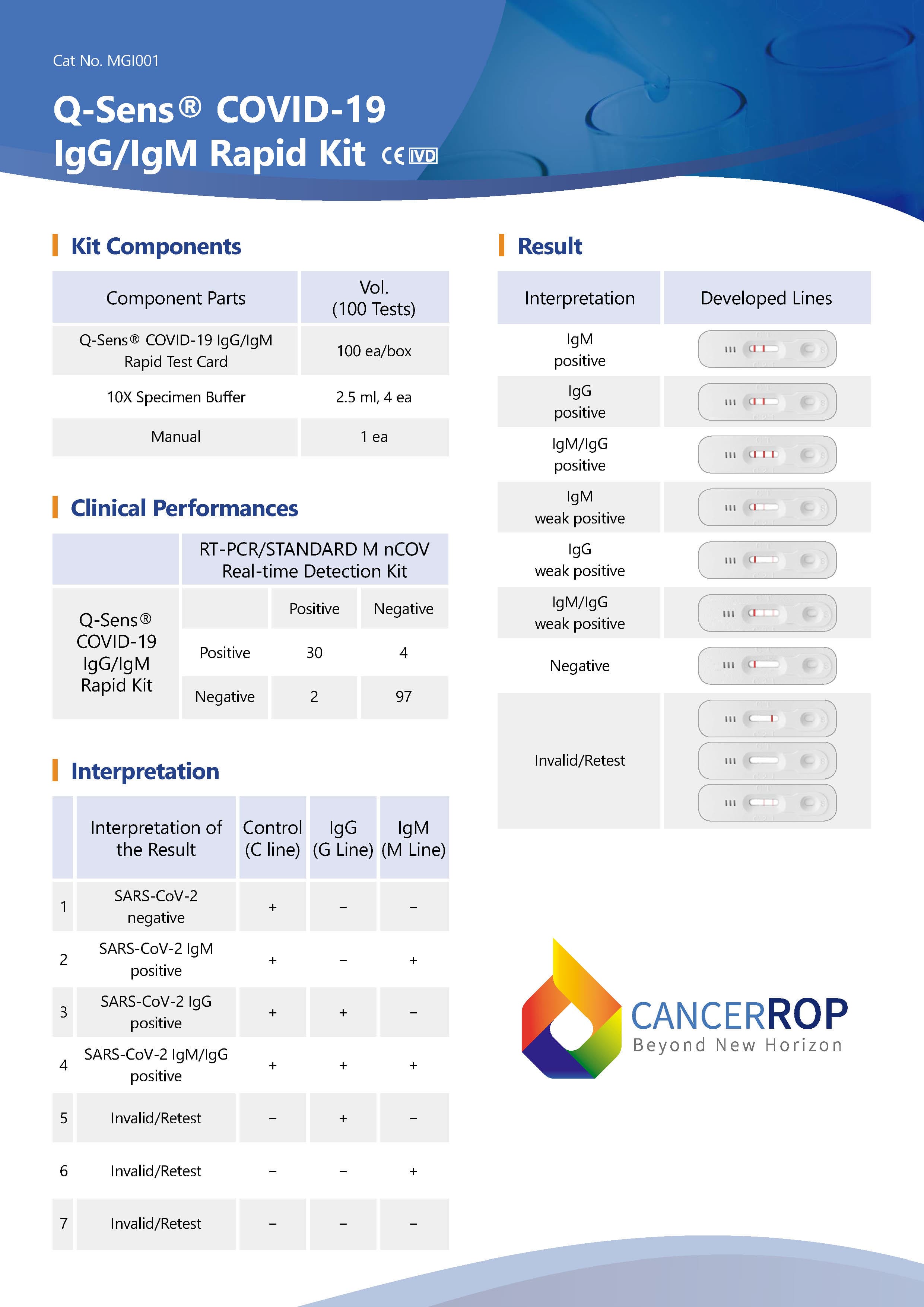
Q-Sens® COVID-19 IgG/IgM Rapid Test Kit
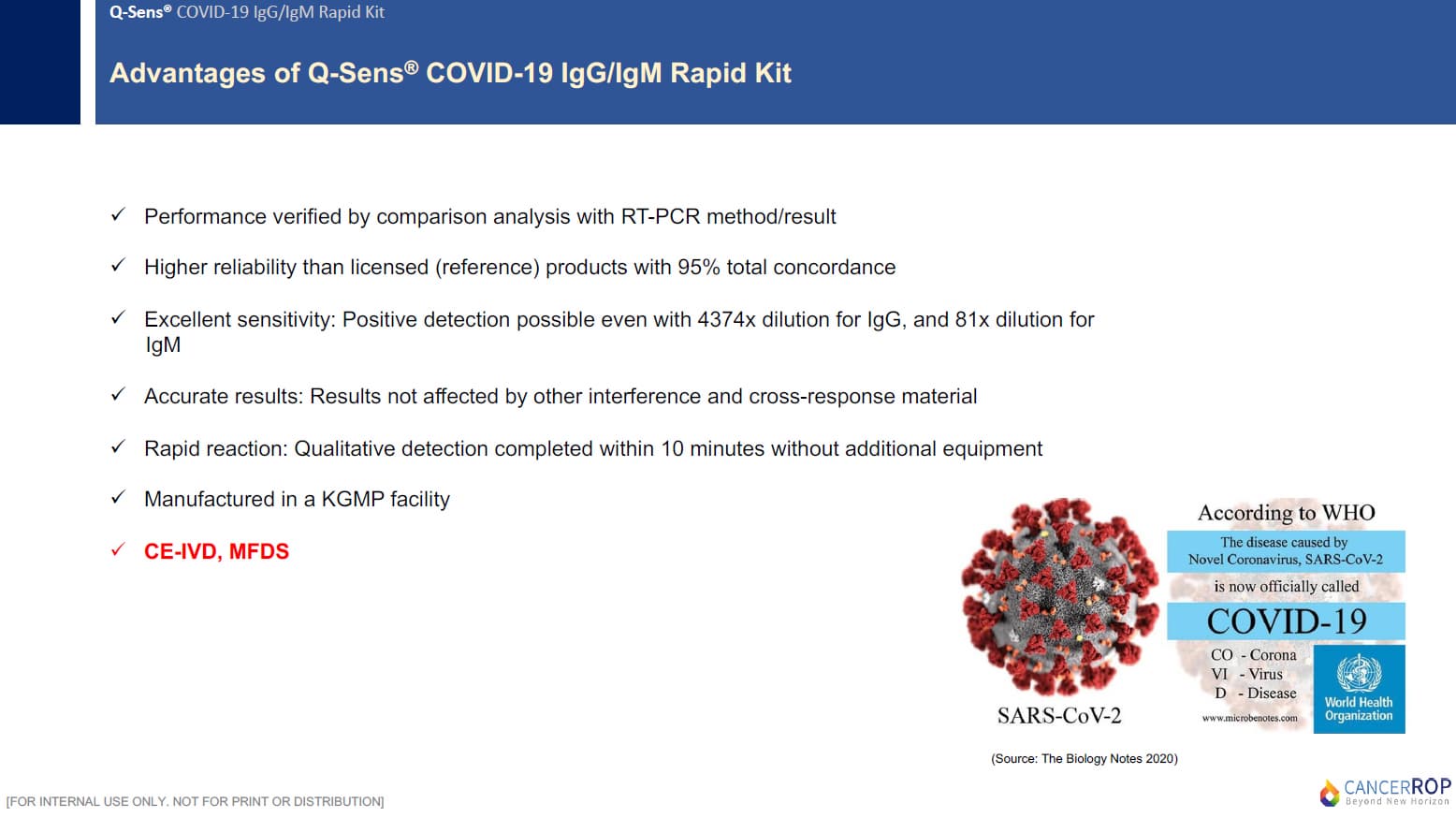
2-2. PRODUCT FEATURES
- Main Markets
-
 Argentina
Argentina
 Brazil
Brazil
 Czecho Republic
Czecho Republic
 Germany
Germany
 Italy
Italy
 Saudi Arabia
Saudi Arabia
 Spain
Spain
 U.A.E.
U.A.E.
- Main Product